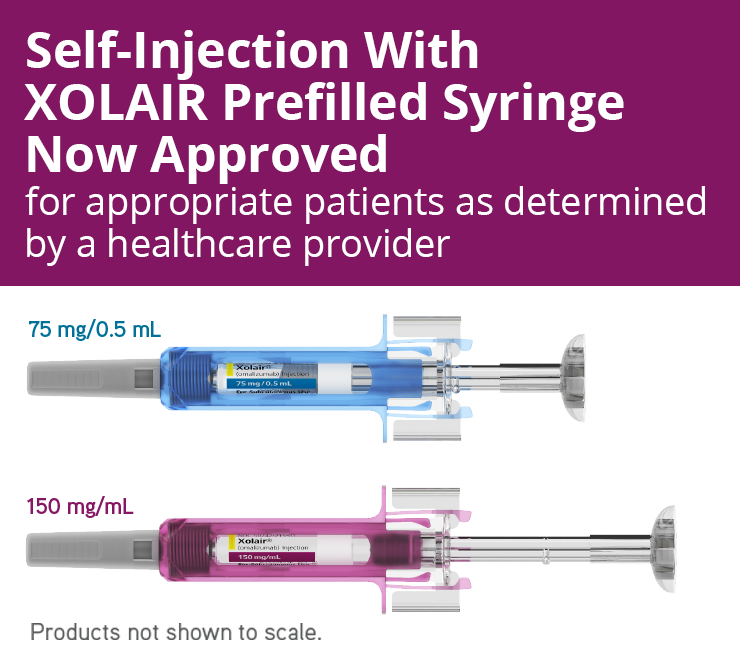
Last month I went to. Xolair omalizumab is administered as a subcutaneous SC injection and is available in prefilled syringes.
 Omalizumab Xolair Page 1 Line 17qq Com
Omalizumab Xolair Page 1 Line 17qq Com
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and who have reduced lung function FEV1.

Who makes xolair. Xolair is produced by a Chinese hamster ovary cell suspension culture. 68 of those users who reviewed Xolair reported a positive effect while 17 reported a negative effect. XOLAIR omalizumab is a treatment for nasal polyps allergic asthma chronic idiopathic urticaria CIU.
Tanox had started developing Xolair and development was completed in collaboration with Novartis and Genentech. Xolair is the first IgE blocker for moderate to severe persistent allergic asthma Xolair acts early in the allergic-inflammatory process in people with allergic asthma. Were self employed with no benefits.
See full prescribing safety Boxed Warning info. For US Healthcare Professionals. Initiate XOLAIR only in a healthcare setting by healthcare providers prepared to manage anaphylaxis that can be life-threatening.
Be sure to review the full Prescribing Information including Medication Guide before administering XOLAIR. Xolair is a drug made by Genentech and marketed in the US. What makes Xolair different.
A skin or blood test is performed to see if you have allergies to year-round allergens. Do not take Xolair if you are having an asthma attack. Jag blir mer eller mindte som en zombie och sover ca 15 timmar under fem sex dagar efter behandlingen.
Xolair is produced by a Chinese hamster ovary cell suspension culture in a nutrient medium containing the antibiotic gentamicin. My friend joked that perhaps the whole concept of Xolair is to make people to tired to do anything so they couldnt over exert themselves and have asthma problems. Food and Drug Administration FDA approved it for treating asthma in 2003 and in 2014 expanded toe approval to include the treatment of chronic hives.
XOLAIR omalizumab for subcutaneous use is an injectable prescription medicine used to treat moderate to severe persistent asthma in people 6 years of age and older whose asthma symptoms are not well controlled with asthma medicines called inhaled corticosteroids. It is made by Genentech Inc. Customers can purchase XOLAIR through authorized specialty distributors and wholesalers that have made a commitment to product integrity.
By Novartis Pharmaceuticals used for the treatment of certain allergic conditions including asthma. It is also approved to treat people with chronic hives without a known cause. An injection at a mere 25000year.
OkI made that one upbut it didnt work either About 8 months ago my Lung Specialist who I have a mad crush on now suggested Xolair. The antibody has a molecular weight of approximately 149 kiloDaltons. In March 2009 Roche acquired Genentech by buying shares it didnt already control for approximately 468 billion.
The acquisition allowed Genentech to keep more of the revenue. For more information on anaphylaxis read Boxed WARNING. Learn about XOLAIR omalizumab a treatment option for chronic idiopathic urticaria patients who remain symptomatic despite H1 antihistamine treatment.
These partners have agreed to distribute only products purchased directly from Genentech and Novartis AG and not to distribute XOLAIR. Xolair is taken by injection. Xolair works by helping to block IgE from causing the reactions that can lead to asthma attacks and symptoms.
Xolair is a recombinant DNA-derived humanized IgG1κ monoclonal antibody that selectively binds to human immunoglobulin E IgE. See full safety boxed warning. Eat 14 cupcakes a day.
Xolair is intended to treat people whose asthma is not controlled with inhaled corticosteroids. The antibody has a molecular weight of approximately 149 kiloDaltons. 1 Use a rescue inhaler short-acting beta agonist instead.
Learn about side effects warnings dosage and more. Work out like crazy build your lung capacity. Xolair is a brand-name prescription drug thats used to treat certain forms of allergic asthma and chronic hives.
 Xolair Omalizumab For Allergic Asthma Nasal Polyps Ciu Treatment
Xolair Omalizumab For Allergic Asthma Nasal Polyps Ciu Treatment
 Xolair Dosage Rx Info Uses Side Effects
Xolair Dosage Rx Info Uses Side Effects
 Xolair Uses Heart Attacks Other Side Effects And Fda Actions
Xolair Uses Heart Attacks Other Side Effects And Fda Actions
 Xolair Omalizumab Allergic Asthma Ciu Nasal Polyps Treatment
Xolair Omalizumab Allergic Asthma Ciu Nasal Polyps Treatment
 Xolair Omalizumab For Allergic Asthma Nasal Polyps Ciu Treatment
Xolair Omalizumab For Allergic Asthma Nasal Polyps Ciu Treatment
Https Www Xolairhcp Com Content Dam Gene Xolairhcp Pdfs Xolair Allergic Asthma Treatment Guide Patients Pdf
 How Xolair May Work To Treat Allergic Asthma Xolair Omalizumab
How Xolair May Work To Treat Allergic Asthma Xolair Omalizumab
 Xolair Omalizumab For Allergic Asthma Nasal Polyps Ciu Treatment
Xolair Omalizumab For Allergic Asthma Nasal Polyps Ciu Treatment
 Xolair Ciu Treatment Steps Xolair Omalizumab
Xolair Ciu Treatment Steps Xolair Omalizumab
 Xolair Omalizumab Allergic Asthma Ciu Nasal Polyps Treatment
Xolair Omalizumab Allergic Asthma Ciu Nasal Polyps Treatment
 Nice Wants More Info On Novartis Xolair In Chronic Hives Pmlive
Nice Wants More Info On Novartis Xolair In Chronic Hives Pmlive
 Xolair Omalizumab For Allergic Asthma Nasal Polyps Ciu Treatment
Xolair Omalizumab For Allergic Asthma Nasal Polyps Ciu Treatment
 What Is Xolair Allergic Asthma Treatment Xolair Omalizumab
What Is Xolair Allergic Asthma Treatment Xolair Omalizumab
 Nasal Polyp Indication Will Help Keep Ageing Xolair Relevant
Nasal Polyp Indication Will Help Keep Ageing Xolair Relevant
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.